Press release -
Investigating protein dynamics: research breakthrough could result in new cancer treatments
Researchers from Northumbria University in Newcastle, and Bogazici University in Turkey, have discovered a new way of examining the structure and dynamics of single protein molecules within the human body – which could help scientists better understand the progression and possible treatment of diseases such as cancer.
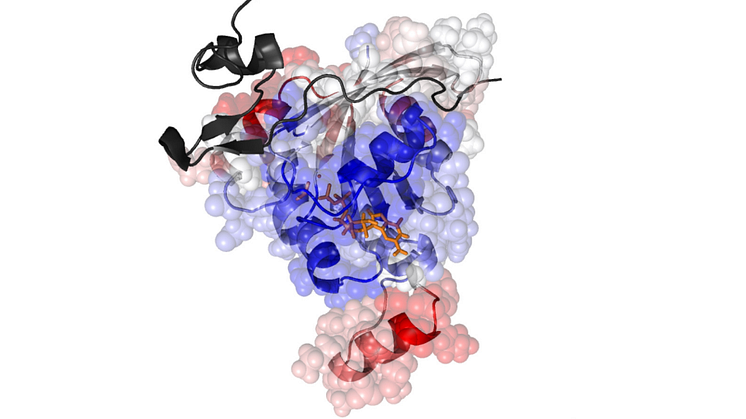
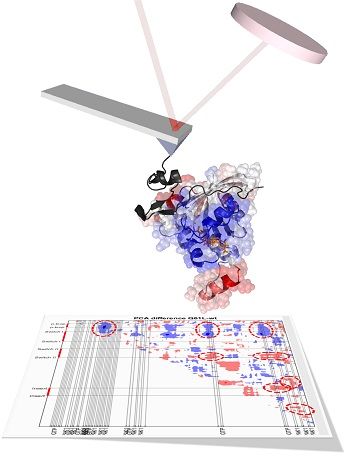
The team, led by Dr Hamdi Torun of Northumbria's Department of Mathematics, Physics and Electrical Engineering, used a technique known as atomic force microscopy which allowed them to identify a single protein molecule within a sample of millions and investigate how it behaved when combined with other molecules.
They combined this technique with a computational investigation led by Professor Turkan Haliloglu, which allows scientists to develop models and simulations-based data – in this case predicting how a molecule might react and mutate following biochemical changes and how this might affect the progression of a disease.
Being able to identify such mutations would allow scientists to understand mutation-led diseases such as cancer and cystic fibrosis, as well as design more effective drugs to treat or vaccinate against these diseases, and even Covid-19 in the future.
Speaking about the research, Dr Torun said: “This is the first time that these two techniques of experimental investigation and computational investigation have been combined to examine a specific protein molecule.
“Isolating one particular molecule in this way is not common practice, but it is the most effective way to probe the structure of the molecule and ask questions such as ‘what if I change a specific part of the protein?’ or ‘what if I attach a specific enzyme to the protein?’
“It is the answers to these questions which will allow us to then design new drugs to treat diseases such as cancer, potentially making a huge difference to people’s lives.”
The research has been published in the Biophysical Journal in a paper entitled Oncogenic mutations on Rac1 affect global intrinsic dynamics underlying GTP and PAK1 binding.
An image depicting the results of their computational investigation was also selected to appear on the front cover ofthe journal (volume 120, issue 5).
The research project, entitled Force and Function in Biological Macromolecules: Molecular Simulation and Single-Molecule Studies, was funded by the Scientific and Technological Research Council of Turkey (TUBİTAK).
Speaking about the potential to use this technique more widely Dr Torun said: “For this study we chose to investigate a protein which is available in all human cells, but the technique could be applied to any protein, for example the spike protein found in Coronavirus.
“This research has huge potential for application, and we hope it will lead to new breakthroughs in the treatment of diseases in the future.”
Find out more about Northumbria University’s Department of Mathematics, Physics and Electrical Engineering.
Topics
Categories
Northumbria is a research-rich, business-focused, professional university with a global reputation for academic excellence. Find out more about us at www.northumbria.ac.uk --- Please contact our Media and Communications team at media.communications@northumbria.ac.uk with any media enquiries or interview requests ---