Press release -
Breathing new life into disease diagnosis
New research shows 100 per cent effectiveness of an innovative breath sampling device developed by Northumbria University medtech spinout, PulmoBioMed, that could revolutionise diagnosis of a range of diseases, including COVID-19.
The results mean that PulmoBioMed’s game-changing device, PBM-HALE™, can be used to study infections of the deep lung, reducing the need for invasive or costly procedures often required for disease diagnosis.
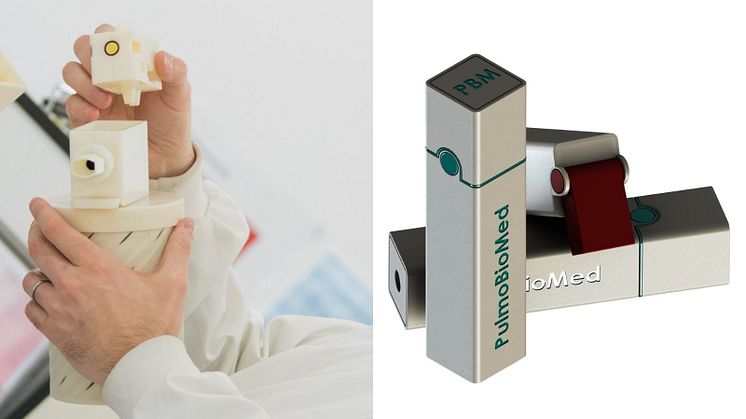
PBM-Hale™ is a hand-held aerosol collecting device that allows sampling of the lung in a non-invasive way - by patients simply breathing into it.
To date, all inventions used for collecting breath samples are challenged by issues relating to contamination, sample loss and variability. PBM-Hale™ technology resolves these issues, with newly published data showing that the device detected 100 per cent of COVID-19 cases tested in the company’s latest clinical study.
Dr Sterghios Moschos, Associate Professor at Northumbria University and Founder and Chief Scientific Officer of PulmoBioMed, said: “We and others have now shown with various devices that exhaled breath contains the virus that causes COVID-19. Previous studies though could not distinguish between what is coming out of the mouth (saliva) and what comes out in fine aerosol particles. Some of them were also very easy to contaminate with virus in the atmosphere or the skin.
“Our study is the first to show that the virus can be detected in the fine aerosol particles generated in the lung, even in resting breath. It shows the virus is found mostly in the tiniest particles we breathe out, not the saliva that might be accidentally spit out as we breathe or speak.
“This means that using our device in combination with the standard PCR test used worldwide, we can detect the genome of the virus in 100% of patients, when sampling the breath for just one minute. This would identify anyone infectious or shedding virus.
“We are also the first to show that the amount of virus produced is 90 times higher on average if you sing loudly. It reinforces why high-quality masks are important to prevent transmission indoors, and why ventilating enclosed spaces is critical to preventing transmission. These small particles are known to linger for hours if the space is not ventilated.
“The study shows that PBM-HALE™ can be used to study infections of the deep lung with no need for invasive procedures that present high risk to the patient and cost thousands to healthcare systems - all in a simple, hand-held device.”
Findings from the study come as the company announces the appointment of world-leading clinical respiratory scientist Professor Peter J Barnes FRS, FMedSci as its new clinical non-executive director. Ranked the most highly cited clinical scientist in the UK and the most highly cited respiratory researcher world-wide for the past 20 years, Professor Barnes has published over 1,000 peer-reviewed papers on asthma, COPD and other airways disease and serves on the editorial boards of over 30 academic journals.
Speaking about PulmoBioMed’s technology, Professor Barnes said: “I am very impressed by the capabilities of the new PBM-HALE™ device, which is able to detect the virus that causes COVID-19 in the breath for the first time. This device has enormous potential application in diagnosing lung infections and in the diagnosing and monitoring of lung diseases, including asthma and COPD. The ease of collecting breath samples non-invasively means that it can be applied to children and to frail and ill patients.”
The PulmoBioMed team presented their latest research at the European Respiratory Society (ERS) International Congress in Barcelona earlier this month.
The preprint paper, detailing the team’s findings and entitled ‘Airborne Pathogen Detection in Fine Aerosol Exhaled Breath Condensates’ and a full list of authors can be found on the health sciences preprint website Medrxiv.
Northumbria University has recently gained reinforcement of its already outstanding reputation for excellence in research, having been ranked 8th in the UK for research power for professions allied to health, such as cellular and molecular sciences, in the latest Research Excellence Framework (REF) 2021.
Taking innovative technology to market
The process of taking academic technology to create PulmoBioMed was made possible by support from Northern Accelerator, a collaboration between Northumbria, Durham, Newcastle and Sunderland Universities to commercialise research and boost the region’s economy.
Northern Accelerator’s ‘Proof-of-Concept’ funding supported the development of the first functional prototype. Northumbria graduate, Saqib Ali, was appointed as a Design Engineer at PulmoBioMed and carried out the rapid prototyping of PBM-HALE™ using 3D printers within the University’s engineering labs.
A second Northern Accelerator initiative, ‘Executives into Business’, supported the onboarding of the executive team, and a third programme of support, ‘Future Founders’, provided business training.
PulmoBioMed also benefitted from North by Northwest Partner’s ‘Innovation to the Commercialisation of University Research’ (ICURe) programme, which helped validate the market for the spinout’s technology. The company won additional funding from Innovate UK to support the first 18 months of business development activities.
For a more detailed explanation of the PBM-HALE™ device, you can watch the video explainer on Northumbria's Vimeo channel.
- Ends –
Notes to editors:
About Professor Peter Barnes
Ranked the most highly cited clinical scientist in the UK and the most highly cited respiratory researcher world-wide for the past 20 years, Professor Barnes has published over 1,000 peer-reviewed papers on asthma, COPD and other airways disease and serves on the editorial boards of over 30 academic journals. He is recognised as the world-leading authority in respiratory disease having been an integral part of the Scientific Committee of Global Guidelines on Asthma (GINA) and COPD (GOLD), and senior author of the American Thoracic Society and European Respiratory Society guidelines and technical standards on Exhaled Breath biomarkers use in lung disease in 2005 and 2017. He was president of the European Respiratory Society in 2013/4, the recipient of 4 honorary MD degrees, delivered multiple prestigious lectures, and the first respiratory physician to attain Royal Society Fellowship in 150 years. Professor Barnes co-founded RespiVert, now acquired by Johnson & Johnson and serves of the Scientific Advisory Board of GSK, AstraZeneca, Boerhinger Ingelheim, Millenium Pharmaceuticals, and the FeNO measuring medical device company Aerocrine.
About PulmoBioMed
PulmoBioMed is a Northumbria University medtech spinout company, founded by Associate Professor Dr Sterghios Moschos. Dr Moschos' pioneering work in biomedical sciences and proof-of-concept funding from Northern Accelerator has led to the creation of an innovative, hand-held breath testing device PBM-Hale™. The device allows sampling of the lungs in a non-invasive way which can be used to detect biomarkers in the breath, for lung conditions such as COVID-19, therapeutic drugs and substances of abuse.
For more information visit www.pulmobiomed.com
Topics
Categories
Northumbria is a research-intensive modern university with a global reputation for academic excellence. Find out more about us at www.northumbria.ac.uk --- Please contact our Media and Communications team at media.communications@northumbria.ac.uk with any media enquiries or interview requests ---